Abstract
Case Report
Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma
Konstantinos Kyratzoglou* and Katie Morton
Published: 07 April, 2025 | Volume 9 - Issue 1 | Pages: 001-003
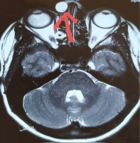
Cystoid Macular Oedema (CMO) is a condition characterized by fluid accumulation in the macular region of the retina, leading to the formation of cyst-like spaces. This edema often results in visual impairment and is associated with various ocular and systemic conditions, including surgery, inflammation, or medication use.
The authors present a case where Cystoid Macular Oedema (CMO) occurred after commencing topical bimatoprost in a pseudophakic patient with primary open angle glaucoma. The macular oedema was treated effectively with a combination of non-steroidal and steroidal topical drops. This case report shows a possible correlation between bimatoprost and CMO, in a patient with no recent confounding risk factors known to contribute to CMO . The recommendation from this report is that all patients treated with topical bimatoprost drops should have a baseline macula OCT examination and a repeated OCT examination 8 weeks after initiation of treatment, to facilitate early detection of CMO.
Read Full Article HTML DOI: 10.29328/journal.ijceo.1001059 Cite this Article Read Full Article PDF
Keywords:
Bimatoprost; Primary open angle glaucoma; Pseudophakic; Cystoid macular oedema
References
- Wagner IV, Stewart MW, Dorairaj SK. Updates on the diagnosis and management of glaucoma. Mayo Clin Proc Innov Qual Outcomes. 2022;6(6):618–635. Available from: https://doi.org/10.1016/j.mayocpiqo.2022.09.007
- National Institute for Health and Care Excellence (NICE). Glaucoma: Diagnosis and Management [Internet]. NICE guideline NG81; 2017 [updated 2022 Jan 26]. Available from: https://www.nice.org.uk/guidance/ng81
- Awwad AM, El Sobky HM, Mandour SS. Study of the effect of prostaglandin analogs on the Periorbita of glaucomatous patients. Menoufia Med J. 2022;35(4):2027. Available from: https://doi.org/10.4103/mmj.mmj_255_22
- Katsanos A, Riva I, Bozkurt B, Holló G, Quaranta L, Oddone F, et al. A new look at the safety and tolerability of prostaglandin analogue eyedrops in glaucoma and ocular hypertension. Expert Opin Drug Saf. 2021;21(4):525–539. Available from: https://doi.org/10.1080/14740338.2022.1996560
- Schilling S, Brobst C, Joondeph BC. An elusive case of cystoid macular edema. Retina Today [Internet]. 2021 Apr:50–51. Available from: https://retinatoday.com/articles/2021-apr/an-elusive-case-of-cystoid-macular-edema
- Zhou Y, Bicket AK, Marwah S, Stein JD, Kishor KS. Incidence of acute cystoid macular edema after starting a prostaglandin analog compared with other classes of glaucoma medications. Ophthalmol Glaucoma. 2025;8(1):4–11. Available from: https://doi.org/10.1016/j.ogla.2024.07.010
- Hu J, Vu JT, Hong B, Gottlieb C. Uveitis and cystoid macular oedema secondary to topical prostaglandin analogue use in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2020;104(8):1040–1044. Available from: https://doi.org/10.1136/bjophthalmol-2019-315280
- Reddy S, Sahay P, Padhy D, Sarangi S, Suar M, Modak R, et al. Tear biomarkers in Latanoprost and bimatoprost treated eyes. PLoS One. 2018;13(8):e0201740. Available from: https://doi.org/10.1371/journal.pone.0201740
Figures:

Figure 1

Figure 2
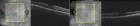
Figure 3
Similar Articles
-
Alternative treatment methods in eyes with pseudophakic cystoid macular edemaAyse Gul Kocak Altintas*. Alternative treatment methods in eyes with pseudophakic cystoid macular edema . . 2019 doi: 10.29328/journal.ijceo.1001019; 3: 001-007
-
Changes in intraocular pressure after ND-yag laser posterior capsulotomyHashim Thiab Hassan*. Changes in intraocular pressure after ND-yag laser posterior capsulotomy. . 2020 doi: 10.29328/journal.ijceo.1001029; 4: 021-030
-
Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle GlaucomaKonstantinos Kyratzoglou*,Katie Morton. Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma. . 2025 doi: 10.29328/journal.ijceo.1001059; 9: 001-003
Recently Viewed
-
Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle GlaucomaKonstantinos Kyratzoglou*,Katie Morton. Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma. Int J Clin Exp Ophthalmol. 2025: doi: 10.29328/journal.ijceo.1001059; 9: 001-003
-
Metastatic Brain Melanoma: A Rare Case with Review of LiteratureNeha Singh,Gaurav Raj,Akshay Kumar,Deepak Kumar Singh,Shivansh Dixit,Kaustubh Gupta*. Metastatic Brain Melanoma: A Rare Case with Review of Literature. J Radiol Oncol. 2025: doi: ; 9: 050-053
-
Depression as a civilization-deformed adaptation and defence mechanismBohdan Wasilewski*,Olha Yourtsenyuk,Eugene Egan. Depression as a civilization-deformed adaptation and defence mechanism. Insights Depress Anxiety. 2020: doi: 10.29328/journal.ida.1001013; 4: 008-011
-
Drinking-water Quality Assessment in Selective Schools from the Mount LebanonWalaa Diab, Mona Farhat, Marwa Rammal, Chaden Moussa Haidar*, Ali Yaacoub, Alaa Hamzeh. Drinking-water Quality Assessment in Selective Schools from the Mount Lebanon. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001061; 8: 018-024
-
Rapid Microbial Growth in Reusable Drinking Water BottlesQishan Liu*,Hongjun Liu. Rapid Microbial Growth in Reusable Drinking Water Bottles. Ann Civil Environ Eng. 2017: doi: 10.29328/journal.acee.1001007; 1: 055-062
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."