More Information
Submitted: 07 May 2020 | Approved: 19 May 2020 | Published: 20 May 2020
How to cite this article: Hamurcu MS, Aydogmuş SK, SARICAOĞLU MS. Evaluation of the efficacy of transcorneal electric stimulation therapy in retinitis pigmentosa patients with electrophysiological and structural tests. Int J Clin Exp Ophthalmol. 2020; 4: 031-037.
DOI: 10.29328/journal.ijceo.1001030
ORCiD : orcid.org/0000-0003-1116-9250
Copyright Licence: © 2020 Hamurcu MS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Flash electroretinogram; Retinitis pigmentosa; Transcorneal electrical stimulation; Visual acuity; Visual evoked potential
Abbreviations: RP: Retinitis Pigmentosa; BCVA: Best Corrected Visual Acuity; TES: Transcorneal Electrical Stimulation; Ferg: Flash Electroretinogram; VF: Visual Field; IOP: Intraocular Pressure; CMT: Central Macular Thickness; RNFLT: Retinal Nerve Fiber Layer Thickness: CT: Choroidal Thickness: Pvep: Pattern Visual Evoked Potential: HK: Hawlina, Konec Loop Electrodes: EPT: Electrical Phosphene Thresholds: USB: Universal Serial Bus Stick: DTL: Dawson, Trick And Litzkow: RGC: Retinal Ganglion Cells: AION: Anterior Ischemic Optic Neuropathy: RCS: Royal College Of Surgeons: IGF-1: Insulin-Like Growth Factor 1: RPE: Retinal Pigment Epithelium
Evaluation of the efficacy of transcorneal electric stimulation therapy in retinitis pigmentosa patients with electrophysiological and structural tests
Mualla Sahin Hamurcu1*, Sema Akkan Aydogmuş2 and M. Sinan SARICAOĞLU3
1Associate Professor, Ankara City Hospital, Department of Ophthalmology, Ankara, Turkey
2Department of Ophthalmology, Ankara City Hospital, Ankara, Turkey
3Professor, Ankara City Hospital, Department of Ophthalmology, Ankara, Turkey
*Address for Correspondence: Mualla Sahin Hamurcu, Ankara City Hospital, Department of Ophthalmology, Ankara, Turkey, Email: [email protected]
A Statement of significance: This study shows that the effect of transcorneal electrical stimulation (TES) therapy as a stimulator device in retinitis pigmentosa (RP)patients with have a significant increase in visual acuity and shortening of p100 latency in pattern visual evoked potential (pVEP) test during 3 months follow up.
Purpose: To assess the safety and efficacy of TES therapy with electrophysiological and structural tests in RP patients.
Methods: Thirty four eyes of 17 RP patients were included in the study. Initial examination included best corrected visual acuity (BCVA) and visual field (VF) test (Humphrey). Central macular thickness (CMT), retinal nerve fiber layer thickness (RNFLT) and choroidal thickness (CT) were measured with using swept-source optical coherence tomography (OCT). The patients were tested by Metrovision brand monpack model visual eletrophysiology device for pVEP and flash electroretinogram (fERG) tests. Patients were seen 12 times during 3 months: initial visit for screening and weekly visits for TES. All tests were repeated 3 times. The results of pre and post TES therapy were compared.
Results: Patients’ baseline BCVA was 0,34 ± 0,22. The increase in the last visit BCVA was significant (p : .001) and it was 0.50 ± 0.29. The difference between CMT, RNLF and CT pre and post TES therapy were not significant (p > .05). The mean latencies of the 120’ pattern p100 waves that patients could see were shortened and statistically significant (p = .04). The peaks amplitudes of the 120’ pattern p100 waves that patients could see were increased; but not statistically significant (p :. 19).
Conclusion: This study shows that the safety of TES as a stimulator device in our patient group and the effect on this group have a significant increase in visual acuity and shortening of p100 latency in pVEP test during 3 months follow up.
Retinitis pigmentosa (RP) is a hereditary disease that results in the alteration of more than 50 genes. These genes carry the instructions for making proteins that are needed in cells within the retina, called photoreceptors. Some of the changes, or mutations, within genes are so severe that the gene cannot make the necessary protein and limit the function of the cells. Other mutations produce a protein that is toxic to the cell, resulting in an abnormal protein that does not function properly. In all cases, the result is damage to the photoreceptors.
For this reason, RP is a group of hereditary disorders characterized by progressive peripheral vision loss and night vision difficulty (nyctalopia), which can lead to central vision loss [1-4].
In the early stages of RP, rods are more affected than cones. As the rods die, people experience night blindness and a progressive loss of the visual field, the area of space that is visible at a given instant without moving the eyes. The loss of rods eventually leads to a breakdown and loss of cones. In the late stages of RP, as cones die, people tend to lose more of the visual field, developing tunnel vision. RP patients may have difficulty performing basic tasks such as daily life, unaided walking, driving [1,2].
Electrical stimulation is a promising therapeutic tool of treatment for a variety of neurological diseases, such as stroke, ear tinnitus and hyperalgesia [1]. A large number of animal experiments have indicated a positive effect of electrostimulation on photoreceptors and ganglion cells in degenerative and traumatic ophthalmic pathologies. Electrostimulation has a long history in ophthalmology and was thought to be beneficial in 1873 [5]. Transcorneal electrical stimulation (TES) has been used for the treatment of amblyopia and amauroses, for retino-choroiditis with pigment infiltration, glaucoma and optic atrophy [2]. TES has been shown in many studies to have positive effects on patients with retinitis pigmentosa, ischemic optic neuropathy, traumatic optic neuropathy and retinal artery occlusions with insignificant complications [5-8].
While the effects of electrostimulation are unclear, different theories have been proposed. In general there are five theories: vasodilatory mechanism, neurotrophic mechanism, antiapoptotic mechanism, antiglutamate mechanism and antiinflammatory mechanism [9,10]. In this study, we aimed to compare clinical and laboratory findings (electrophysiological and structural tests) pre and post TES therapy in patients with retinitis pigmentosa.
This prospective study was performed in Ankara Numune Training and Research Hospital, Turkey. Thirty four eyes of 17 patients who were diagnosed with retinitis pigmentosa included between May 2017 and January 2018. Ethics Committee Approval was taken. The study was conducted in accordance with the principles of Declaration of Helsinki. All participants provided their informed consents and current status, natural course, treatment success rates and risks.
The following selection criteria were used.
Inclusion criteria were age 18 to 60 years, best corrected visual acuity (BCVA) 0.05 to 0.9 (decimal notation), recordableβlash electroretinogram (fERG ) tests (> 5 μV in amplitude), and reliable visual βield (VF) results (> 150°2 in area). Exclusion criteria were other ocular diseases than RP (e.g., glaucoma, diabetic retinopathy, exudative age-related macular degeneration, history of retinal detachment, macular edema, retinal or choroidal neovascularization, mental retardation, pregnancy, or severe systemic disease such as epilepsy, cerebrovascular disease history, uncontrolled hypertension, hearth and kidney disease
Written consent was obtained from the patients. Initial examination included BCVA with decimal notation, intraocular pressure (IOP) measurement, light reβlex, relative afferent pupillary defect, color discrimination examination, visual βield (VF) and examination of the anterior and posterior segments, followed by swept- source optical coherence tomography (OCT) (3D OCT-1000 Mark II, Topcon, Japan). 30-2 VF (Humprey) was performed to the patients as the location of visual βield defects varied. The changes in the visual βieldbefore and after treatment of each patient were compared and reported whether there was any improvement. Central macular thickness (CMT), retinal nerve βiber layer thickness (RNFLT) and choroidal thickness (CT) were measured with using OCT. BCVA was measured in each eye using the Early Treatment of Diabetic Retinopathy Study (ETDRS; Lighthouse International, New York, NY, USA) three-chart series at three meters.
In accordance to International Society for Clinical Electrophysiology of Vision (ISCEV) standards [11], the patients were tested by Metrovision brand monpack model visual electrophysiology device for pattern visual evoked potential (pVEP) and βlash electroretinogram (fERG) tests [11].PVEP is made simultaneously, using high-contrast (80%) checkerboard stimuli subtending the visual arc (min arc) with varying patterns. Tests were done with 120’ pattern size. Retinal and visual pathway functions were assessed by single βlash ERG test. Rod response (25 db) b wave amplitude (μV) and cone response b wave amplitude were compared. Hawlina, Konec (HK) loop electrodes were used for fERG tests.
A commercially available stimulation system was used consisting of OkuStim, OkuSpex, and OkuEl (CE approved; Okuvision GmbH, Reutlingen, Germany). OkuStim is the stimulation unit that delivers pulses of 20 Hz with current-balanced 5 ms positive immediately followed by 5 ms negative deβlections. Only the study team had a software to determine electrical phosphene thresholds (EPT) and upload stimulation parameters for home use onto a patient’s individual Universal Serial Bus (USB) stick; the patient used the USB stick to startstimulation by plugging it into the OkuStim. The USB stick recorded time, date, electrical parameters, and duration of stimulation until the next visit to the study center. During stimulation the device checked the impedance of the attached electrodes and alerted when impedance was too high. OkuSpex is the special frame to be adjusted to the patient’s face and to accept the electrodes. OkuEl are the electrodes based on the Dawson, Trick and Litzkow (DTL) type described originally by Dawson, et al. [12]. The electrodes have been constructed to be positioned on the conjunctival inner face of lower eyelid when the patient is wearing the lens frame. A red dot electrodefrom 3M (3M Europe, Diegem, Belgium) was attached to the ipsilateral temple as counter electrode.
All of the patients were treated in Electrophysiology Department on the given appointment day. Patients were dropped propacain HCL 0.5% (Alcaine, Alcon) before the procedure. TES was performed for 30 minutes per week for 12 consecutive weeks. Both eyes were stimulated during TES. The stimulation strength was adjusted at each visit according to the individual EPT.
Patients were called for evaluation on another day before and after treatment. They were seen 12 times during 3 months: initial and last visit for screening (visit 1 for BCVA, IOP, VF, OCT) and weekly visits for TES. All tests were repeated 3 times. Theresults of pre and post TES therapy were compared.
Statistical analysis was performed using the IBM SPSS for Windows version 23.0 software (IBM Corp., Armonk, NY). Descriptive statistics were expressed in mean ±standard deviation (SD) and range (min-max) values. Variance analysis was used for repeated measures for the variables with normal distribution in time (ANOVA), and the Friedman test for the normal non-distributed variables. When the difference was signiβicant in the Friedman test; Dunn’s multiple comparison test was used in the bilateral encounters in time. p value of < .05 was considered statistically signiβicant.
Seventeen participants, with clinically conβirmed RP were recruited into an open-label observational trial from Ankara Numune Training and Research Hospital, Turkey. Thirty-four eyes of seventeen patients, 6 women (35.3%) and 11 men (64.7%) comprised the study group in this prospective study. The mean age of patients was 39.53 ± 11.99 (range, 19-59 years). In the ophthalmologic examination of patients one patient had exotropia. They had no systemic diseases.
Seventeen participants completed the initial 3-month treatment period. The treatment protocol was tolerated well. No serious adverse events or study dropouts related to the treatment were observed. Patient compliance was well.
Patients’ baseline mean BCVA was 0.34 ± 0.22 (decimal notation). Following the treatment period, mean BCVA increased to 0.35 ± 0.21 in the βirst month, 0.45 ± 0.28 in thesecond month and 0.50 ± 0.29 in the third month. The increase in last visit BCVA (3 months following initiation of TES was statistically signiβicant (p = .001) (Table 1).
| Table 1: The change of parameters in the study, pre and post TES therapy. | |||||
| Variables | Baseline (mean ± Sd) |
1st Month (mean ± Sd) |
2nd Month (mean ± Sd) | 3rd Month (mean ± Sd) |
p |
| BCVA | 0.34 ± 0.22 | 0.35 ± 0.21 | 0.45 ± 0.28 | 0.50 ± 0.29 | 0.00 |
| CMT(μ) | 213 ± 37 | 218 ± 29 | 217 ± 36 | 230 ± 41 | 0.121 |
| RLNF(μ) | 72 ± 30 | 67 ± 28 | 72 ± 28 | 68 ± 26 | 0.19 |
| CT(μ) | 252 ± 86 | 262 ± 74 | 249 ± 70 | 256 ± 76 | 0.71 |
| pVEP 120' P100 amplitude (μ V) | 3.77 ± 2.54 | 4.16 ± 2.31 | 4.21 ± 2,49 | 4.46 ± 2.65 | 0.19 |
| pVEP 120' p100 latencies (ms) | 120.9 ± 26.1 | 122.7 ± 12 | 121 ± 15 | 120 ± 12 | 0.04 |
| pVEP - smallest pattern size | 60 | 60 | 30 | 30 | 0.00 |
| ERG rod response (25db) b wave amplitude (μ V) | 20.5 ± 28 | 22 ± 28 | 22.4 ± 30 | 22.2 ± 28.2 | 0.08 |
| ERG cone response b wave amplitude (μ V) | 7.6 ± 6 | 8.3 ± 6 | 8.6 ± 6 | 9.6 ± 7 | 0.11 |
| BCVA: Best Corrected Visual Acuity; CMT: Central Macular Thickness; RNLF: Retinal Nerve Layer Fiber; CT: Choroidal Thickness; pVEP: Pattern Visual Evoked; Potential; ERG: Electroretinography; Sd: Standart deviation; TES: Transcorneal Electrical Stimulation | |||||
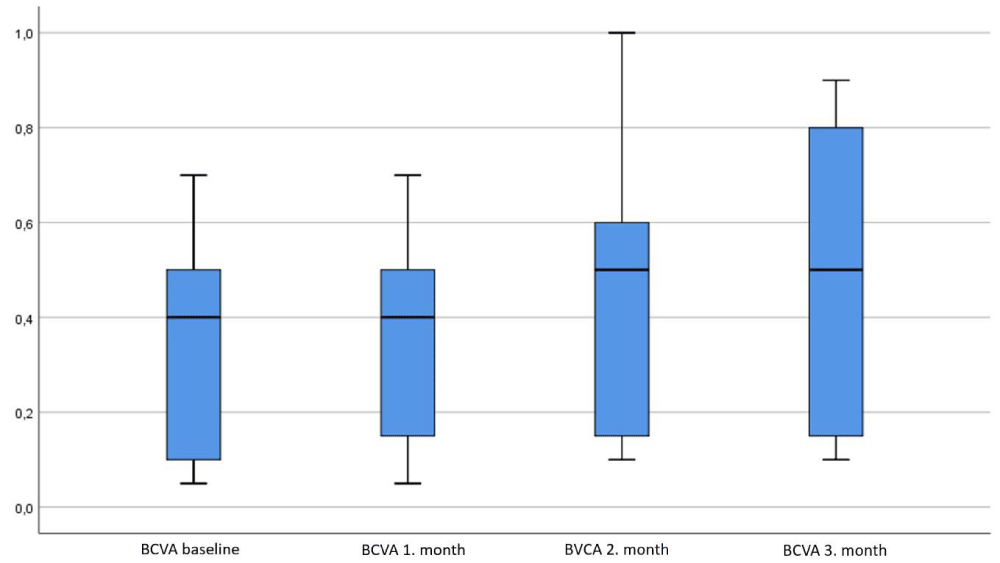
Figure 1 shows that the distribution of BCVA in the pre-treatment, 1-month, 2-month and 3-month, minimum-maximum values and median values. Also all BCVA of all patients are present in the table 2.
Figure 1: The change in visual acuty during treatment.
| Table 2: Patients’ BCVA, pre and post TES therapy. | ||||
| Number of eyes | BCVA pre-treatment | BCVA 1st month | BCVA 2nd month | BCVA 3rt month |
| 1 | 0.7 | 0.7 | 0.8 | 0.8 |
| 2 | 0.5 | 0.4 | 0.6 | 0.6 |
| 3 | 0.5 | 0.5 | 0.4 | 0.5 |
| 4 | 0.5 | 0.5 | 0.4 | 0.6 |
| 5 | 0.5 | 0.5 | 0.6 | 0.8 |
| 6 | 0.5 | 0.5 | 0.9 | 0.9 |
| 7 | 0.4 | 0.4 | 0.6 | 0.6 |
| 8 | 0.5 | 0.5 | 0.5 | 0.6 |
| 9 | 0.1 | 0.1 | 0.1 | 0.1 |
| 10 | 0.1 | 0.1 | 0.1 | 0.1 |
| 11 | 0.1 | 0.15 | 0.15 | 0.2 |
| 12 | 0.05 | 0.05 | 0.1 | 0.1 |
| 13 | 0.3 | 0.5 | 0.5 | 0.6 |
| 14 | 0.05 | 0.05 | 0.15 | 0.2 |
| 15 | 0.05 | 0.15 | 0.1 | 0.1 |
| 16 | 0.1 | 0.15 | 0.15 | 0.15 |
| 17 | 0.4 | 0.4 | 0.5 | 0.6 |
| 18 | 0.4 | 0.3 | 0.6 | 0.6 |
| 19 | 0.05 | 0.05 | 0.1 | 0.1 |
| 20 | 0.05 | 0.1 | 0.1 | 0.1 |
| 21 | 0.3 | 0.3 | 0.4 | 0.5 |
| 22 | 0.3 | 0.3 | 0.4 | 0.5 |
| 23 | 0.4 | 0.6 | 0.7 | 0,9 |
| 24 | 0.4 | 0.6 | 0.6 | 0,8 |
| 25 | 0.2 | 0.2 | 0.6 | 0.8 |
| 26 | 0.2 | 0.3 | 0.2 | 0.5 |
| 27 | 0.1 | 0.15 | 0.15 | 0.15 |
| 28 | 0.15 | 0.15 | 0.15 | 0.15 |
| 29 | 0.7 | 0.7 | 0.8 | 0.8 |
| 30 | 0.4 | 0.4 | 0.5 | 0.5 |
| 31 | 0.5 | 0.6 | 0.6 | 0.8 |
| 32 | 0.6 | 0.7 | 0.6 | 0.5 |
| 33 | 0.7 | 0.7 | 1.0 | 0.9 |
| 34 | 0.7 | 0.5 | 1.0 | 0.9 |
| BCVA: Best Corrected Visual Acuity; TES: Transcorneal Electrical Stimulation | ||||
There was a signiβicant improvement in visual βield in 28 eyes (82.4%) and no improvement in 6 eyes (17.6%).
The mean latencies (in milliseconds) of the 120’ pattern p100 waves that patients could see were shortened and statistically signiβicant (p = .04). The peaks amplitudes of the 120’ pattern p100 waves that patients could see were increased but not statistically signiβicant (p = .19).
In this study, the smallest pVEP pattern size that patients could see was taken into account. The patients could see smaller patterns in pVEP test with TES therapy and the change was statistically signiβicant (p = .001). When the smallest pattern size that the patient can see with pVEP test is evaluated, the pattern size seen after 2nd and 3rd TES therapy there was a statistically signiβicant decrease when compared with pre-treatment and βirst month (p = .001).
In ERG, there was no change statistically signiβicant in the scotopic rod b-wave (p = .008) and con amplitude (p = .011) in 3 months follow-up period.
RP is a hereditary disorder characterized by progressive photoreceptor degeneration and so far there has been no satisfactory treatment available yet [1-4].
In RP, more than 200 genetic transitions have been identiβied and are increasingly identiβied. Different genetic structures can often have different disease mechanisms that result only in a seemingly similar disease. These different genetic pathomechanisms may impede the disease at different levels or speed in RPE and other retinal cells. So different methods are currently being tried to treat the disease. However, no deβinitive treatment has yet been found to correct or stop deformation in retinal cells.
Electrical stimulation has been tried and promising results in various neurological diseases. Many neurology clinics; have reported their results on laboratory safety and tolerability proβiles in relation to this subject [5.10]. Following neurological use, non-invasive administration in the eye has resulted in positive results on retinal cells. The animal test series have shown that retinal neurons, such as retinal ganglion cells (RGC) and photoreceptors, can protect against traumatic or genetically induced degeneration and improve visual function loss [1-3]. These therapeutic evidence supports the use of ophthalmological treatments against a variety of retinaland optical diseases. The positive results of TES have been published in patients with RP, traumatic optic neuropathy, anterior ischemic optic neuropathy (AION) and retinal artery occlusion [14,15,21,22].TES has a neuroprotective effect on degenerative retinal cells by altering electrical activity or electrical charge balance of photoreceptors. Royal College of Surgeons (RCS), an hereditary RP animal model in which TES enhances the survival of photoreceptors, has been shown to protect retinal function in rats. In this study fundusexamination performed at the end of the experiments; It has been reported that complications such as retinal detachment, vitreous hemorrhage are not seen in eyes treated with TES. Indicating that TES is innocuous to vitreous or retinal tissuesin RP models and provides positive safety proβiles for TES [16]. In our study, in some patients during the sessions, therewere no complications other than ocular discomfort due to the electrodes.
It is thought that the effect of TES is enhanced by increasing the release of neurotrophic agents. In the study by Sato, et al. Rat retina Muller cells show that the enhancement of insulin-like growth factor 1 (IGF-1) transcription by ES in cultured Muller cells depends largely on Ca (2+) inβlux through L- channels. It regenerates at the cellular level by increasing the factors involved in retinal transport. Studies have shown that they increase 25 different proteins in rats. TES had effects onthe expression of retinal proteins. These results will contribute to our knowledge on the mechanism of how TES affects the retina [17,18].
Kurimato, et al. reported increased chorioretinal circulation after TES [19], but it is unclear whether this is in addition or secondary to neurotrophic upregulation.
Improvement in visual acuity after TES administration; it is suggested that the neuroprotective effect caused by the treatment in the retinal cells that have not completely lost the function provides regeneration in the retinal cells. Thus, enlargement in the narrowed visual βield can be attributed to the stimulation of cells that did not completely lose their vitality in peripheral retina. Consistent with previous studies, visual acuity increased in our patient group [20-22]. Wagner, et al. reported an increase in visual acuity but not signiβicant [23]. They reported a visual function measurements at 6 months demonstrated no signiβicant difference between the controls and treated eyes [23].
In RP cases, different types of visual βield defects can be detected depending on the distribution of affected cells in the retina. In visual βield testing, peripheral constriction iscommon and can progress to tunnel vision [1-3].There was a statistically signiβicant improvement in visual βield defectsafter TES in our study; it may seem that regional improvements in the rod and cones provide enlargement of the narrowed visual βields.
ERG provides evaluation of the occipital cortex visual system from the retinal pigment epithelium (RPE) by recording the common electrical response produced by neural and non-neuronal cells in the retina. ERG is a mass response and is therefore normal when the dysfunction is limited to small retinal areas [11].
The pVEP measures the cortical cells response against pattern stimuli. In this study, pVEP was used to objectively evaluate the improvement in VA and to show changes in p100 latency and amplitude.
This test is routinely used in the objective measurement of the VA in our Electrophysiology laboratory. In pVEP, different pattern sizes are used in the checkerboard pattern and the amplitudes and latencies of the p100 response are evaluated. In this study, changes in amplitude and latency before and after treatment were compared in 120 ‘and the smallest seen patterns. The pattern sizes are determined based on the angle of each pattern with the fovea when the patient is looking at the screen. P100 values obtained from the age-matched normal population, which are deβined in our laboratory in accordance with the ISCEV standards, are used [11]. The VA is reported according to the response in the smallest pattern by examining the morphology and amplitude values of the mentioned pattern size. p 100 latency, which we found to be signiβicant in the study, shows that shorter stimulus and neural activity are transmitted faster.
Weakening in retinal vessels, photoreceptor cell loss and deterioration of metabolic requirement due to degeneration in retinal ganglion cells are responsible for ethiopathogenesis [4].It has been reported in the literature that the neuroprotectiveeffect of TES reverses the optic nerve dysfunction due to regeneration in retinal ganglion cells [13]. In our study, statistically signiβicant shortening in the duration of p100 latency with VEP 120’ pattern suggests that transmission is accelerating. The smallest pattern size seen by the patients decreased statistically signiβicantly in the last two controls compared to the βirst two months. This explains why patients’ treatments have begun to see smaller patterns in the last two months.
In a study of TES on rabbits, the healing effect on cone function could be demonstrated by fERG. In previous studies, only scotopic ERG was reported to be signiβicantly improved in the treated group, and improvement in cone cells on rabbits could also be shown. It has been reported that photoreceptors can be protected from degeneration by stimulating aerobic glycolysis with TES [14].VF improvement and limited or improved resolution of cone and rod responses in ERG supported the assumption of beneβicial effects on the cones and supported the idea that both visual βield size and cone ERG were signiβicantly related to Schatz, et al. tendencies of improved function were observed for scotopic b-wave amplitude [21]. In the same author’s work in 2017, signiβicant improvement of light-adapted single βlash b-wave was noted and tendencies of improved function were observed for scotopic b-wave amplitude were noted [22]. The reason for the not signiβicant changes in the rods and cones in our patient group, fERG is the record of a diffuse electrical response generated by neural and non-neuronal cells within the retina. Therefore, the local improvement in rod and cone responses in our patient group could not be determined by fERG. In our patient group, the initial rod and cone b wave amplitudes in the most of patients were quite low. RP disease duration suggests that in the long term, there is no response to remission regarding retinal cell involvement.
Bittner, et al. published a series of cases, followed by an article in which they compared retinal blood βlow and visual function changes with different techniques after TES treatment. They reported increased blood βlow following electrostimulation therapies as an objective, physiological improvement in addition to visual function improvements in some RP patients [24,25].
OCT is a commonly used method in non-invasive and easy-to-use ophthalmology practice. The OCT helps the clinician to offer optical biopsy of the tissues. Provides cross-sectional display of retinas in vivo using reβlection of light waves. RNFLT may be evaluated by assessing optic reβlective differences among the retinal layers [26]. It enables morphometric and quantitative measurement of retina and optic nerve, and hence may be used in the diagnosis and follow up of diseases. In our treatment group, the difference between CMT, RNLF and CT before and after treatment were not statistically signiβicant. This may be due to the fact that structural rehabilitation is more difβicult than functional recovery in patients with long-term exposure to disease, or that the duration of treatment we are taking is short.
Patients’ visual acuity and visual βield improved. However, the changes in the rod and cone b wave potentials in the ERG and OCT were not signiβicant, indicating that there was no structural improvement despite the improvement in the function of the retinal cells.
This study shows that the safety of TES as a stimulator device in our patient group and the effect on this group have a signiβicant increase in visual acuity and shortening of p100 latency in pVEP test during 3 months follow up.
Although we achieved positive results in TES treatment in our clinic, we had limitations. Because we could not use a test that would evaluate the visual βield more concrete and duration was not long enough.
And there are still restrictions on TES therapy. It is not clear how the treatment protocol is deβinitive and how long the duration of action will last. For this reason, we think of TES as a potential treatment in patients with RP, but we think thatthe details that need to be met with the work to be done.
- Heckenlively JR. Retinitis Pigmentosa. Philadelphia: JB Lippincott. 1988.:
- Hartong DT, Berson EL, Dryja TP. Retinitis Pigmentosa. Lancet. 2006; 368: 1795–809. PubMed: https://pubmed.ncbi.nlm.nih.gov/17113430/
- He Y, Zhang Y, Su G. Recent Advances in Treatment of Retinitis Pigmentosa. Curr Stem Cell Res Ther. 2015; 10: 258-265. PubMed: https://pubmed.ncbi.nlm.nih.gov/25345673/
- Grunwald JE, Maguire AM, Dupont J. Retinal Hemodynamics in Retinitis Pigmentosa. Am J Ophthalmol. 1996; 122: 502-508. PubMed: https://pubmed.ncbi.nlm.nih.gov/8862046/
- Gekeler F, Bartz-Schmidt KU. Electrical stimulation-a therapeutic strategy for retinal and optic nerve disease? Graefes Arch Clin Exp Ophthalmol. 2012; 2: 161-163. PubMed: https://pubmed.ncbi.nlm.nih.gov/22282217/
- Fujikado T, Morimoto T, Matsushita K, Shimojo H, Okawa Y, et al. Effect of Transcorneal Electrical Stimulation in Patients with Nonarteritic Ischemic Optic Neuropathy or Traumatic Optic Neuropathy. Jpn J Ophthalmol. 2006; 50: 266–273. PubMed: https://pubmed.ncbi.nlm.nih.gov/16767383/
- Fedorov A, Chibisova Y, Szymaszek A, Alexandrov M, Gall C, et al. Non-invasive Alternating Current Stimulation Induces Recovery From Stroke. Restor Neurol Neurosci. 2010; 28: 825–833. PubMed: https://pubmed.ncbi.nlm.nih.gov/21209497/
- Tao Y, Chen T, Liu B, Peng GH, Qin LM, et al. The Transcorneal Electrical Stimulation as a Novel Therapeutic Strategy Against Retinal and Optic Neuropathy: a Review of Experimental and Clinical Trials. Int J Ophthalmol. 2016; 9: 914-919. PubMed: https://pubmed.ncbi.nlm.nih.gov/27366697/
- De Santana JM, Walsh DM, Vance C, Rakel BA, Sluka KA, et al. Effectiveness of Transcutaneous Electrical Nerve Stimulation for Treatment of Hyperalgesia and Pain. Curr Rheumatol Rep. 2008; 10: 492–499. PubMed: https://pubmed.ncbi.nlm.nih.gov/19007541/
- Kleinjung T, Steffens T, Londero A, Langguth B. Transcranial Magnetic Stimulation (TMS) for Treatment of Chronic Tinnitus: Clinical Effects. Prog Brain Res. 2007; 166: 359–367. PubMed: https://pubmed.ncbi.nlm.nih.gov/17956800/
- Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, et al. ISCEV Standard for Clinical Visual Evoked Potentials. Doc Ophthalmol. 2016; 133: 1-9. PubMed: https://pubmed.ncbi.nlm.nih.gov/27443562/
- Dawson WW, Trick GL, Litzkow CA. Improved Electrode for Electroretinography. Invest Ophthalmol Vis Sci. 1979; 18: 988. PubMed: https://pubmed.ncbi.nlm.nih.gov/478786/
- Ma Z, Cao P, Sun P, Li L, Lu Y, et al. Optical Imaging of Visual Cortical Responses Evoked by Transcorneal Electrical Stimulation with Different Parameters. Invest Ophthalmol Vis Sci. 2014; 55: 5320-5331. PubMed: https://pubmed.ncbi.nlm.nih.gov/25082881/
- Inomata K, Shinoda K, Ohde H, Tsunoda K, Hanazono G, et al. Transcorneal Electrical Stimulation of Retina to Treat Longstanding Retinal Artery Occlusion. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 1773–1780. PubMed: https://pubmed.ncbi.nlm.nih.gov/17593383/
- Gekeler F, Messias A, Ottinger M, Bartz-Schmidt KU, Zrenner E. Phosphenes Electrically Evoked with DTL Electrodes: a Study in Patients with Retinitis Pigmentosa, Glaucoma, and Homonymous Visual Field Loss and Normal Subjects. Invest Ophthalmol Vis Sci. 2006; 47: 4966–4974. PubMed: https://pubmed.ncbi.nlm.nih.gov/17065515/
- Morimoto T, Fujikado T, Choi JS, Kanda H, Miyoshi T, et al. Transcorneal Electrical Stimulation Promotes the Survival of Photoreceptors and Preserves Retinal Function in Royal College of Surgeons Rats. Invest Ophthalmol Vis Sci. 2007; 48: 4725-4732. PubMed: https://pubmed.ncbi.nlm.nih.gov/17898297/
- Sato T, Fujikado T, Lee TS, Matsushita K, Harada T, et al. Direct Effect of Electrical Stimulation on Induction of Brain-derived Neurotrophic Factor from Cultured Retinal Muller cells. Invest Ophthalmol Vis Sci. 2008; 49: 4641-4646. PubMed: https://pubmed.ncbi.nlm.nih.gov/18661273/
- Sato T, Fujikado T, Lee TS, Matsushita K, Harada T, et al. Effect of Electrical Stimulation on IGF-1 Transcription by L-type Calcium Channels in Cultured Retinal Muller cells. Jpn J Ophthalmol. 2008; 52: 217-223. PubMed: https://pubmed.ncbi.nlm.nih.gov/18661273/
- Kurimoto T, Oono S, Oku H, Tagami Y, Kashimoto R, et al. Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin Ophthalmol. 2010; 4: 1441–1446. PubMed: https://pubmed.ncbi.nlm.nih.gov/21188156/
- Robles-Camarillo D, Nino-de-RiveraL, Lopez-Miranda J, et al. The Effect of Transcorneal Electrical Stimulation in Visual Acuity: Retinitis pigmentosa. J Biomedical Sci Engineering. 2013; 6: 1-7.
- Schatz A, Röck T, Naycheva L, Willmann G, Wilhelm B, et al. Transcorneal Electrical Stimulation for Patients with Retinitis Pigmentosa: a Prospective, Randomized, Sham-controlled Exploratory Study. Invest Ophthalmol Vis Sci. 2011; 52: 4485–4496. PubMed: https://pubmed.ncbi.nlm.nih.gov/21467183/
- Schatz A, Pach J, Gosheva M, Willmann G, Wilhelm B, et al. Transcorneal Electrical Stimulation for Patients with Retinitis Pigmentosa: A Prospective, Randomized, Sham-Controlled Follow-up Study over 1 Year. Invest Ophthalmol Vis Sci. 2017; 58: 257-269. PubMed: https://pubmed.ncbi.nlm.nih.gov/21467183/
- Wagner SK, Jolly JK, Pefkianaki M, Gekeler F, Webster AR, et al. Transcorneal Electrical Stimulation for the Treatment of Retinitis Pigmentosa: Results from the TESOLAUK trial. BMJ Open Ophthalmol. 2017; 2 :e000096 PubMed: https://pubmed.ncbi.nlm.nih.gov/29354722/
- Bittner AK, Seger K. Longevity of Visual Improvements Following Transcorneal Electrical Stimulation and Efficacy of Retreatment in Three Individuals with Retinitis Pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2018; 256: 299–306. PubMed: https://pubmed.ncbi.nlm.nih.gov/29222719/
- Bittner AK, Seger K, Salveson R, Kayser S, Morrison N, et al. Randomized Controlled Trial of Electro-Stimulation Therapies to Modulate Retinal Blood Flow and Visual Function in Retinitis Pigmentosa. Acta Ophthalmol. 2018; 96: e366-e376. PubMed: https://pubmed.ncbi.nlm.nih.gov/29130647/
- Mumcuoglu T, Erdurman C, Durukan AK. Principles of optical coherence tomography and application innovations. T Oft Gaz. 2008; 38: 168-175.