More Information
Submitted: March 08, 2025 | Approved: April 04, 2025 | Published: April 07, 2025
How to cite this article: Kyratzoglou K, Morton K. Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma. Int J Clin Exp Ophthalmol. 2025; 9(1): 001-003. Available from:
https://dx.doi.org/10.29328/journal.ijceo.1001059.
DOI: 10.29328/journal.ijceo.1001059
Copyright Licence: © 2025 Kyratzoglou K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bimatoprost; Primary open angle glaucoma; Pseudophakic; Cystoid macular oedema
Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma
Konstantinos Kyratzoglou* and Katie Morton
The Royle Eye Department, Pilgrim Hospital, Boston, UK
*Address for Correspondence: Konstantinos Kyratzoglou, MD, FEBO, The Royle Eye Department, Pilgrim Hospital, Boston, UK, Email: [email protected]
Cystoid Macular Oedema (CMO) is a condition characterized by fluid accumulation in the macular region of the retina, leading to the formation of cyst-like spaces. This edema often results in visual impairment and is associated with various ocular and systemic conditions, including surgery, inflammation, or medication use.
The authors present a case where Cystoid Macular Oedema (CMO) occurred after commencing topical bimatoprost in a pseudophakic patient with primary open angle glaucoma. The macular oedema was treated effectively with a combination of non-steroidal and steroidal topical drops. This case report shows a possible correlation between bimatoprost and CMO, in a patient with no recent confounding risk factors known to contribute to CMO. The recommendation from this report is that all patients treated with topical bimatoprost drops should have a baseline macula OCT examination and a repeated OCT examination 8 weeks after initiation of treatment, to facilitate early detection of CMO.
This case report details the incidence of Cystoid Macular Oedema (CMO) in one eye following commencement of bimatoprost eye drops – a Prostaglandin Analogue (PGA). Pharmacotherapy is the most common choice for lowering intraocular pressure in the management of glaucoma and ocular hypertension and eye drops in the Prostaglandin Analogues (PGAs) are commonly selected as first-line therapy [1]. Despite increased use of laser trabeculoplasty in recent years in the use of selective laser trabeculoplasty as first-line treatment, since the NICE guidelines were updated in 2022, PGAs are a popular alternative for those patients that either cannot tolerate the laser process, are non-responders or have contraindications such as narrow angles or pigment dispersion syndrome [2]. PGAs are known to have side effects but the frequent prescribing of them is due to the benefit of the strong efficacy in lowering intraocular pressure, outweighing the risk. Most side effects are localised and minor such as lash lengthening and hyperpigmentation, iris hyperpigmentation and dry eye symptoms [3,4]. Cystoid macular oedema is a rare but more serious side effect, with the trigger mechanism remaining unknown [5,6]. When latanoprost was approved by the FDA in 1996 as the first topical ocular PGA, CMO was not listed as a possible side effect. However, since then, due to several reports to the FDA ‘Adverse Event Reporting System’ and publications in literature, it is now listed in the medication leaflet. Similarly, it is listed for bimatoprost under ‘uncommon’ potential side effects. A theory is that CMO occurs with this group of medication due to confounding factors which are often cited as potential additional catalysts of CMO [6]. This could be recent cataract surgery, ruptured posterior lens capsule or active inflammatory conditions such as uveitis. This case report describes a patient who developed CMO without any recent confounding factors and the subsequent resolution following additional treatment.
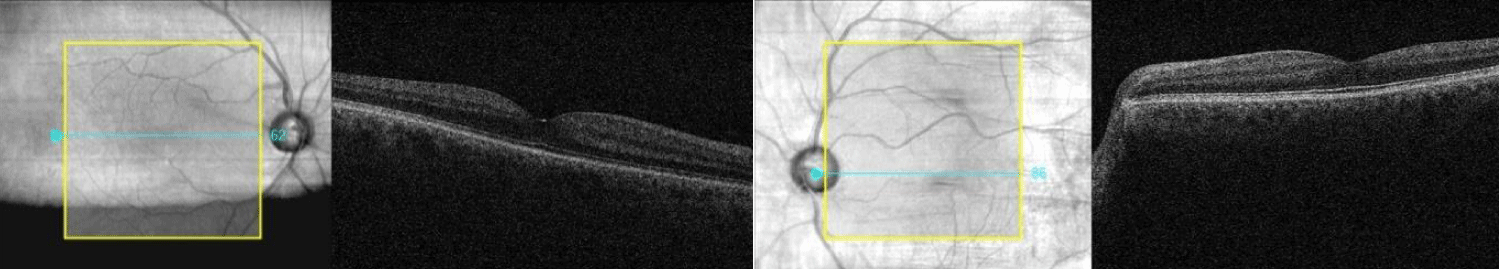
A 54-year-old Caucasian man was referred from his GP to the hospital eye clinic, due to transfer of his care from another area. His ocular history included primary open angle glaucoma treated with dorzolamide/timolol (20 mg/ml + 5 mg/ml) eye drops b.i.d to both eyes, uncomplicated cataract surgery in both eyes 2 years prior and laser retinopexy for a retinal tear in his right eye. The patient reported no systemic health conditions. On examination his visual acuity (VA) was -0.06 logMAR in the right eye and -0.10 in the left and his intraocular pressures (IOPs) were 28 mmHg and 27 mmHg respectively. The cup-to-disc ratio was 0.80 (right eye) and 0.85 (left eye). Visual fields showed glaucomatous defects in both eyes. Baseline OCT examination of the macula (Figure 1) was unremarkable. Due to his suboptimal intraocular pressures, bimatoprost (100 micrograms/ml) eye drops o.d were prescribed as additional treatment in both eyes.
Figure 1: Baseline OCT of the macula (both eyes) showing normal findings.
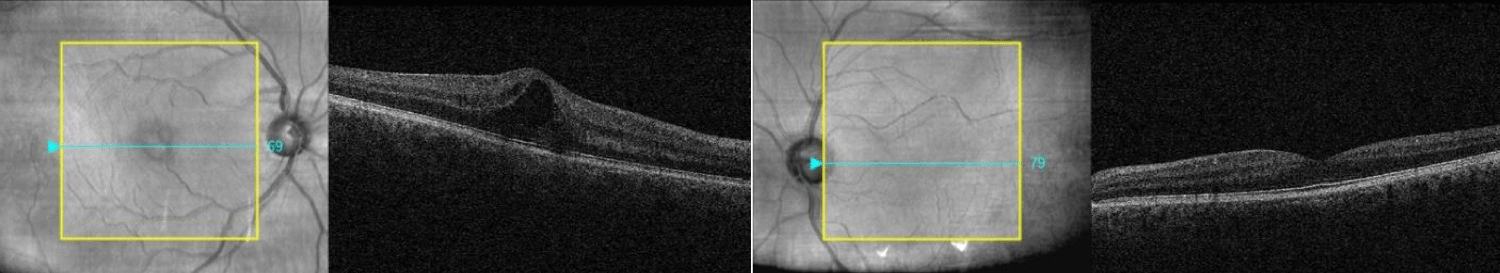
Seven weeks later, the patient re-presented to clinic with complaints of blurred vision in the right eye. VA in the right eye declined to 0.14; left eye VA remained stable at -0.08 logMAR. IOPs were 14 mmHg in the right eye and 17 mmHg in the left. OCT examination showed CMO in the right eye (Figure 2). Bimatoprost drops were discontinued in the right eye. Treatment was initiated with topical bromfenac (900 micrograms/ml) eye drops b.i.d and dexamethasone (0.1%) eye drops t.i.d to his right eye and brimonidine (0.2%) eye drops b.i.d to both eyes.
Figure 2: Cystoid Macular Oedema in the right eye following bimatoprost initiation.
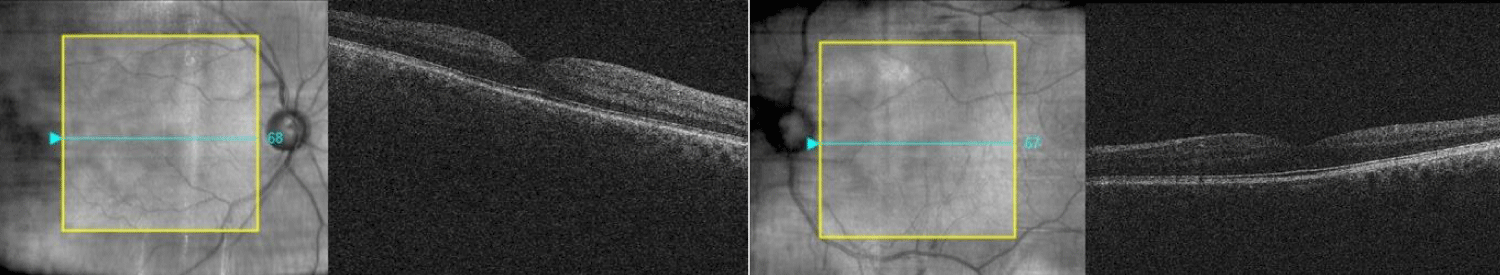
4 weeks later, the patient’s right VA improved to -0.20 (left eye –0.16). OCT examination showed resolution of the CMO in the right eye (Figure 3). The patient admitted administering brimonidine drops to his right eye just one week prior to his appointment. IOPs were 20 mmHg in the right eye and 10 mmHg in the left. The patient is currently monitored for his glaucoma.
Figure 3: Resolution of CMO after discontinuing bimatoprost and initiating topical therapy.
This patient developed CMO within 7 weeks after initiation of treatment with bimatoprost – a prostaglandin analogue. Hu, et al. in 2019, reported the incidence of CMO in bimatoprost users to be 0.01% and the median time of onset to be 30 days after the introduction of PGA [7]. Zhou, et al. in 2024, reported an even greater incidence of 0.13% for CMO in PGA users [6].
Research suggests that topical PGA increases the production of inflammatory mediators [8]. Whether this effect reaches the posterior pole remains debated; however, some researchers suggest transvitreal diffusion and posterior spread of PGA in pseudophakic eyes possibly due to the reduced physical barrier in pseudophakic eyes [7]. Another hypothesis suggests that endogenous prostaglandins that are present after cataract surgery are potentiated by PGAs, causing disruption of the blood-retinal barrier [6]. To the best of the authors’ knowledge, no studies currently examine about laser-treated retinal tears as a risk co-factor for development of CMO, but this should also be considered in this particular patient.
This case report shows a possible correlation between topical bimatoprost and CMO, in a pseudophakic patient. The recommendation from this case report is that all patients started on bimatoprost treatment should undergo baseline and follow-up macular OCT examinations 8 weeks after initiation of treatment, to facilitate early detection of CMO.
- Wagner IV, Stewart MW, Dorairaj SK. Updates on the diagnosis and management of glaucoma. Mayo Clin Proc Innov Qual Outcomes. 2022;6(6):618–635. Available from: https://doi.org/10.1016/j.mayocpiqo.2022.09.007
- National Institute for Health and Care Excellence (NICE). Glaucoma: Diagnosis and Management [Internet]. NICE guideline NG81; 2017 [updated 2022 Jan 26]. Available from: https://www.nice.org.uk/guidance/ng81
- Awwad AM, El Sobky HM, Mandour SS. Study of the effect of prostaglandin analogs on the Periorbita of glaucomatous patients. Menoufia Med J. 2022;35(4):2027. Available from: https://doi.org/10.4103/mmj.mmj_255_22
- Katsanos A, Riva I, Bozkurt B, Holló G, Quaranta L, Oddone F, et al. A new look at the safety and tolerability of prostaglandin analogue eyedrops in glaucoma and ocular hypertension. Expert Opin Drug Saf. 2021;21(4):525–539. Available from: https://doi.org/10.1080/14740338.2022.1996560
- Schilling S, Brobst C, Joondeph BC. An elusive case of cystoid macular edema. Retina Today [Internet]. 2021 Apr:50–51. Available from: https://retinatoday.com/articles/2021-apr/an-elusive-case-of-cystoid-macular-edema
- Zhou Y, Bicket AK, Marwah S, Stein JD, Kishor KS. Incidence of acute cystoid macular edema after starting a prostaglandin analog compared with other classes of glaucoma medications. Ophthalmol Glaucoma. 2025;8(1):4–11. Available from: https://doi.org/10.1016/j.ogla.2024.07.010
- Hu J, Vu JT, Hong B, Gottlieb C. Uveitis and cystoid macular oedema secondary to topical prostaglandin analogue use in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2020;104(8):1040–1044. Available from: https://doi.org/10.1136/bjophthalmol-2019-315280
- Reddy S, Sahay P, Padhy D, Sarangi S, Suar M, Modak R, et al. Tear biomarkers in Latanoprost and bimatoprost treated eyes. PLoS One. 2018;13(8):e0201740. Available from: https://doi.org/10.1371/journal.pone.0201740